Research focus on Cognitive Neurology
Heart & Brain Center Göttingen (HBCG)
The overarching goal of our research program is to understand the neural mechanisms underlying visual consciousness and visuomotor coordination. We aim to bridge the gap between basic neuroscience and clinical research. We have a specific focus on higher-order thalamic nuclei such as the pulvinar and ask how thalamo-cortical interactions contribute to conscious perception and visuomotor behavior. We tackle those questions at different temporal and spatial neural levels, primarily in healthy volunteers, non-human primates and neurological patients. We employ visual psychophysics, multimodal MRI and electrophysiological methods such as EEG-ECG, eye tracking and brain stimulation (e.g. tACS, TMS).
Heart & Brain research is a recent development in our laboratory and some of the main projects are described below.
Heart-Brain Projects: Introduction
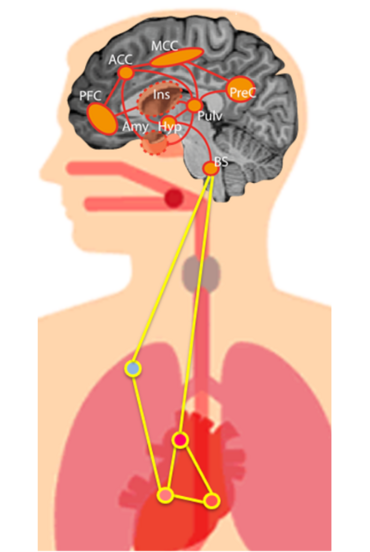
Recent evidence indicates that cortical oscillations and, accordingly, perception and cognition, are shaped by external stimuli and intrinsic neural fluctuations as well as cardiac signals. A possible source of cardiac and neural interactions is a set of brain regions that processes cardiac signals and forms the ‘central autonomic network’ (CAN). These CAN brain regions largely overlap with arousal-related regions, which increase their activity concurrently with heart rate during physical and cognitive (‘autonomic’) challenges. While the location and basic function of the CAN has been established by neuroimaging in recent years, we currently lack a physiological account that can explain how cardiac signals interact with ongoing neural activity.
Our heart-brain research has three major aims:
- Establish a physiological mechanism by which cardiac activity affects neural activity and ensuing visual perception.
- Determine the causal contributions of CAN brain regions in mediating cardiac effects on neural activity
- Testing CAN activity during autonomic challenges as a predictor for cardiac dysfunction in stroke patients.
Influence of cardiac signals on cortical dynamics
We aim to elucidate how activity in CAN regions affects visual perception, thought to be mediated by brain regions that lie far removed from the CAN. Those experiments in healthy human subjects aim to facilitate the development of a physiologically valid model of heart-brain interactions. We test the hypothesis that heart-evoked activity in CAN regions propagates to connected brain regions, e.g. via travelling waves, where it affects ongoing oscillations. To achieve these aims, we employ (i)EEG and brain stimulation (e.g. TMS) methods. We hypothesize that CAN activity propagates to distant yet connected brain regions such as visual cortex in the form of a travelling wave, i.e., a spatio-temporal pattern of oscillatory neural activity that sweeps the cortical surface. We aim to differentiate this possibility from other plausible scenarios, namely that cardiac activity affects perceptual processes by triggering activity in general arousal pathways.
*Funded: by the Else-Kroener-Research Foundation and the GRK 2824
Influence of cardiac signals on conscious visual perception
We aim to probe the ‘causal’ relationship between visual perception and cardiac activity by combining visual illusions and threshold-visual detection tasks with autonomic challenges such as cycling exercise. By simultaneously measuring ECG-EEG and pupil size we test the idea that heart and breathing rates influence perceptual thresholds, while controlling for general arousal effects.
*Funded: by the Else-Kroener-Research Foundation and the GRK 2824
Multimodal analysis of the CAN as a predictor for cardiac dysfunction in stroke patients
(Collaboration with Mathias Bähr, Clinic for Neurology)
Neurological patients often suffer from stroke of unknown sources. However, some patients would develop atrial fibrillation (AF) after the stroke. We test the hypothesis that pathological heart-and-brain interactions, independent of typical stroke risk factors, can predict the development of atrial fibrillation. We assume that CAN activation patterns in stroke patients, e.g. triggered and measured via autonomic challenges can predict AF development and other secondary heart and cognitive problems.
*Funded: by the GRK 2824